Association Between Body Mass Index, Early B-cell Repopulation and Clinical Outcomes in Multiple Sclerosis (P11-1.001)Abbas, Song, Kelly
et alNeurology (2025) 104 (7_Supplement_1), 4229
Abstract: Early B-cell repopulation occurs in people with Multiple Sclerosis (pwMS) receiving B-cell depleting therapies (BCD). There is limited knowledge on the potential predictors of early B-cell repopulation and its clinical implications. We examined the (1) association between body mass index (BMI) and early B-cell repopulation and (2) association between early B-cell repopulation and subsequent clinical outcomes in pwMS on BCD.N/A.We conducted a retrospective study in two academic centers. From pwMS who completed at least one full cycle of BCD (Ocrelizumab or Rituximab), we obtained BMI, CD19%, demographic and clinical features as well as outcomes in relation to BCD administration. We operationally defined early B-cell repopulation as CD19% value of ≥2% within 4.5-6 months following infusion. The primary clinical outcomes included annualized relapse rate and confirmed disability worsening (based on patient determined disease steps). Additional clinical outcomes in subgroup analysis included functional testing scores and optic coherence tomography metrics. We performed covariate-adjusted regression analyses.The study included 293 pwMS (mean age 45.86±12.75 years, 70% women). Higher BMI was significantly associated with greater likelihood of early B-cell repopulation (estimate=1.08, 95%CI [1.05, 1.11], p<0.001) after confounder adjustment. The same association persisted in subgroup analysis examining Ocrelizumab or Rituximab separately. Race/ethnicity was independently associated with early B-cell repopulation, with non-white and/or Hispanic PwMS being 1.40 times more likely to experience early B-cell repopulation than non-Hispanic white pwMS. Early B-cell repopulation was not significantly associated with increased annualized relapse rate or confirmed patient-reported disability progression or worsening of any of the examined secondary MS outcomes.Among pwMS receiving BCD, higher BMI and racial/ethnic minorities are associated with early B-cell repopulation, but early B-cell repopulation is not associated with worse clinical outcomes. Larger prospective studies with longer follow-up duration are warranted to confirm these findings. Disclaimer: Abstracts were not reviewed by Neurology® and do not reflect the views of Neurology® editors or staff. Disclosure: Dr. Abbas has nothing to disclose. Emily Song has nothing to disclose. Dr. Kelly has nothing to disclose. Ms. Osterhaus has received research support from University of Pittsburgh School of Medicine. Dr. Zhu has nothing to disclose. Ms. Son has nothing to disclose. Dr. Riley has received personal compensation in the range of $500-$4,999 for serving as a Consultant for Genentech. Dr. Riley has received personal compensation in the range of $10,000-$49,999 for serving on a Scientific Advisory or Data Safety Monitoring board for EMD Serono. Dr. Riley has received personal compensation in the range of $10,000-$49,999 for serving on a Scientific Advisory or Data Safety Monitoring board for TG Therapeutics. Dr. Riley has received personal compensation in the range of $10,000-$49,999 for serving on a Scientific Advisory or Data Safety Monitoring board for Horizon. Dr. Riley has received personal compensation in the range of $500-$4,999 for serving on a Scientific Advisory or Data Safety Monitoring board for Novartis. Dr. Riley has received personal compensation in the range of $500-$4,999 for serving on a Scientific Advisory or Data Safety Monitoring board for Viracta. Dr. Riley has received personal compensation in the range of $500-$4,999 for serving on a Scientific Advisory or Data Safety Monitoring board for TG Therapeutics. Dr. Riley has received personal compensation in the range of $500-$4,999 for serving on a Scientific Advisory or Data Safety Monitoring board for Roche. Dr. Riley has received personal compensation in the range of $500-$4,999 for serving on a Scientific Advisory or Data Safety Monitoring board for Amgen. Dr. Riley has received personal compensation in the range of $500-$4,999 for serving on a Scientific Advisory or Data Safety Monitoring board for Immunic AG. Dr. Riley has received personal compensation in the range of $500-$4,999 for serving on a Scientific Advisory or Data Safety Monitoring board for Cabaletta Bio. Dr. Riley has received personal compensation in the range of $500-$4,999 for serving on a Scientific Advisory or Data Safety Monitoring board for Bristol Myers Squibb. The institution of Dr. Xia has received research support from National Institute of Health. The institution of Dr. Xia has received research support from Department of Defense. The institution of Dr. Xia has received research support from Genentech/Roche.
An Investigator Initiated Study of KYV-101, a CD19 CAR T Cell Therapy, in Participants with Treatment Refractory Progressive Multiple Sclerosis (S3.002)Gupta, Seshadri, Lincoln
et alNeurology (2025) 104 (7_Supplement_1), 4110
Abstract: This phase 1 study is to document the presence of KYV-101 chimeric antigen receptor (CAR)-T cells in the central nervous system (CNS) through their detection in cerebral spinal fluid (CSF) and surmise depletion of CNS resident B cells via anti-CD19 CAR-T cells through clearance of oligoclonal bands (OCB)s and/or normalization of IgG index in adult participants with treatment-refractory progressive multiple sclerosis (MS).Despite the success of B cell depleting therapies in suppressing disease activity in relapsing forms of MS, many patients with progressive forms of MS continue to experience worsening disability. This may be due to CNS resident B cells that are resilient to peripheral B cell depletion by anti-CD20 monoclonal antibodies. CD19-targeted CAR-T cells offers the promise to deplete B cells in the peripheral immune system and within the CNS.This is an open-label, single ascending dose study of KYV-101, autologous CD3+ T cells genetically engineered to express a fully human, second generation anti-CD19 CAR (KYV-101). Participants will be age 25-70 with treatment refractory progressive MS. Five participants will be at dose-level 1 of 0.33×108 CAR-T cells intravenously after a preconditioning regime. Five other patients (DL2) will receive 1×108 CAR T cells. Key study procedures include CSF, MRI, and monitoring of standard laboratory parameters, neurologic status, patient reported outcomes, and EDSS regularly through the follow up period.A 55 year old female with EDSS 6, was dosed on 8/27/24 without cytokine release syndrome or immune effector cell-associated neurotoxicity syndrome. Further details of her CAR expansion are being analyzed. We will continue enrolling participants and look for safety and tolerability of KYV-101.The present trial will provide preliminary data which we expect will support the use of CD19 CAR T-cell therapy for this disease. All available data will be presented at the time of the conference. Disclaimer: Abstracts were not reviewed by Neurology® and do not reflect the views of Neurology® editors or staff. Disclosure: Dr. Gupta has nothing to disclose. Dr. Seshadri has received personal compensation in the range of $500-$4,999 for serving as a Consultant for Abbvie. Dr. Seshadri has received personal compensation in the range of $500-$4,999 for serving as a Consultant for AstraZeneca. Dr. Seshadri has received personal compensation in the range of $500-$4,999 for serving as a Consultant for BeiGene. Dr. Seshadri has received personal compensation in the range of $500-$4,999 for serving as a Consultant for Kite. The institution of Dr. Seshadri has received research support from Kyverna. Robin R. Lincoln has nothing to disclose. Ms. Okinishi has nothing to disclose. Miss Shum has nothing to disclose. Mrs. Shenoy has received personal compensation in the range of $500-$4,999 for serving as a Consultant for Janssen. Mrs. Shenoy has received personal compensation in the range of $500-$4,999 for serving on a Scientific Advisory or Data Safety Monitoring board for Janssen. Mrs. Wilmoth has received personal compensation in the range of $500-$4,999 for serving on a Speakers Bureau for Gilead. Mrs. Wilmoth has received personal compensation in the range of $500-$4,999 for serving on a Speakers Bureau for Sanofi Genzyme. Dr. Hauser has received personal compensation in the range of $500-$4,999 for serving as a Consultant for NGM Bio. Dr. Hauser has received personal compensation in the range of $500-$4,999 for serving as a Consultant for Moderna. Dr. Hauser has received personal compensation in the range of $500-$4,999 for serving as a Consultant for BD. Dr. Hauser has received personal compensation in the range of $500-$4,999 for serving as a Consultant for Pheno Therapeutics. Dr. Hauser has received personal compensation in the range of $500-$4,999 for serving as a Consultant for Nurix Therapeutics. Dr. Hauser has received personal compensation in the range of $0-$499 for serving on a Scientific Advisory or Data Safety Monitoring board for Accure. Dr. Hauser has received personal compensation in the range of $10,000-$49,999 for serving on a Scientific Advisory or Data Safety Monitoring board for Alector. Dr. Hauser has received personal compensation in the range of $0-$499 for serving on a Scientific Advisory or Data Safety Monitoring board for Annexon. Dr. Hauser has received personal compensation in the range of $10,000-$49,999 for serving on a Scientific Advisory or Data Safety Monitoring board for Hinge Therapeutics. Dr. Hauser has received personal compensation in the range of $10,000-$49,999 for serving as an officer or member of the Board of Directors for Neurona. Dr. Hauser has a non-compensated relationship as a Clinical Trial/Primary Investigator with Roche that is relevant to AAN interests or activities. Dr. Hauser has a non-compensated relationship as a Clinical Trial/Primary Investigator with Novartis that is relevant to AAN interests or activities. The institution of Dr. Cree has received personal compensation in the range of $5,000-$9,999 for serving as a Consultant for Biogen. The institution of Dr. Cree has received personal compensation in the range of $10,000-$49,999 for serving as a Consultant for EMD Serono. The institution of Dr. Cree has received personal compensation in the range of $10,000-$49,999 for serving as a Consultant for Novartis. The institution of Dr. Cree has received personal compensation in the range of $10,000-$49,999 for serving as a Consultant for Sanofi. The institution of Dr. Cree has received personal compensation in the range of $10,000-$49,999 for serving as a Consultant for TG Therapeutics. The institution of Dr. Cree has received personal compensation in the range of $500-$4,999 for serving as a Consultant for Autobahn. The institution of Dr. Cree has received personal compensation in the range of $500-$4,999 for serving as a Consultant for Avotres. The institution of Dr. Cree has received personal compensation in the range of $500-$4,999 for serving as a Consultant for Alexion. Dr. Cree has received personal compensation in the range of $10,000-$49,999 for serving as a Consultant for Horizon. Dr. Cree has received personal compensation in the range of $10,000-$49,999 for serving as a Consultant for Neuron23. Dr. Cree has received personal compensation in the range of $500-$4,999 for serving as a Consultant for Boston Pharma. Dr. Cree has received personal compensation in the range of $500-$4,999 for serving as a Consultant for Hexal/Sandoz. Dr. Cree has received personal compensation in the range of $500-$4,999 for serving as a Consultant for Kyverna. Dr. Cree has received personal compensation in the range of $500-$4,999 for serving on a Scientific Advisory or Data Safety Monitoring board for Immunic AG. The institution of Dr. Cree has received research support from Genentech. The institution of Dr. Cree has received research support from Kyverna. Dr. Cree has received publishing royalties from a publication relating to health care.
Project EVOLVE: An international analysis of postimmunotherapy lineage switch, an emergent form of relapse in leukemiaSilbert, Rankin, Hoang
et alBlood (2025)
Abstract: Lineage switch (LS), defined as the immunophenotypic transformation of acute leukemia, has emerged as a mechanism of relapse following antigen-targeted immunotherapy which is associated with dismal outcomes. Through an international collaborative effort, we identified cases of LS following a host of antigen-targeted therapies (e.g., CD19, CD22, CD38 and CD7), described how LS was diagnosed, reviewed treatment approaches, and analyzed overall outcomes for this form of post-immunotherapy relapse. Collectively, 75 cases of LS were evaluated, including 53 (70.7%) cases of B-ALL to AML, 17 (22.7%) cases of B-ALL to mixed phenotypic acute leukemia (MPAL)/acute leukemias of ambiguous lineage (ALAL), and 5 (6.7%) cases of rare LS presentation (i.e., T-cell ALL to AML). An additional 10 cases with incomplete changes in immunophenotype, referred to as "lineage drift" were also described. With a primary focus on the 70 cases of LS from B-ALL to AML or MPAL/ALAL, LS emerged at a median of 1.5 months (range, 0-36.5 months) post-immunotherapy, with 81.4% presenting with LS within the first 6 months from the most proximal immunotherapy. While the majority involved KMT2A rearrangements (n=45, 64.3%), other rare cytogenetic and/or molecular alterations were uniquely observed. Treatment outcomes were generally poor with < 40% remission rates. The median overall survival following LS diagnosis was 4.8 months. Outcomes were similarly poor for those with rare immunophenotypes of LS or "lineage drift." This global initiative robustly categorizes lineage changes post-immunotherapy and, through enhanced understanding, establishes a foundation for improving outcomes of LS.Copyright © 2025 American Society of Hematology.
Impact of complement C3 levels on the development of healthcare-associated infections in intensive care patients: a retrospective case-control studyWang, Chen, Ying
et alAnn Med (2025) 57 (1), 2487631
Abstract: The immune system serves as a critical line of defence against pathogenic microorganisms. To investigate the impact of immune markers, measured within the first 48 h of intensive care unit (ICU) admission, on the incidence of healthcare-associated infections (HAIs) in ICU patients.This case-control study included 364 patients admitted from 1 January 2020 to 30 November 2023, receiving immune marker testing within 48 h of ICU admission. Cox proportional hazard models and propensity score matching evaluated immune markers' association with HAIs risk. Log-rank tests compared time-to-event by C3 levels. All data processing and analysis were performed using R version 4.2.0 (R Foundation for Statistical Computing, Vienna, Austria) and Python version 3.11 (Python Software Foundation, Wilmington, DE).In total, 258 patients without HAIs (mean [SD] age, 67.24 [17.79] years) and 106 patients with HAIs (mean [SD] age, 73.80 [14.93] years) were included in the final analysis. The HAIs group had older age, longer hospital stay, lower Sequential Organ Failure Assessment (SOFA) scores, and a higher rate of comorbid infections than the non-HAIs group. Also, the HAIs group had a higher proportion of basophils, lymphocytes, monocytes and T suppressor cells (CD3 + CD8+), while the proportion of neutrophils and B cells (CD19+) was lower. After Cox regression analysis and propensity score adjustment, we found that C3 complement levels (HR: 0.40; 95%CI, 0.16-0.98; p = .044) influenced the incidence of HAIs. Patients were then divided into high C3 and low C3 groups based on a cut-off value of 0.455 for C3. A time-to-event plot showed that the median time to HAIs occurrence was nine days in the high C3 group and six days in the low C3 group (p = .048).Elevated complement C3 levels may associat with a reduced incidence of HAIs in ICU patients.


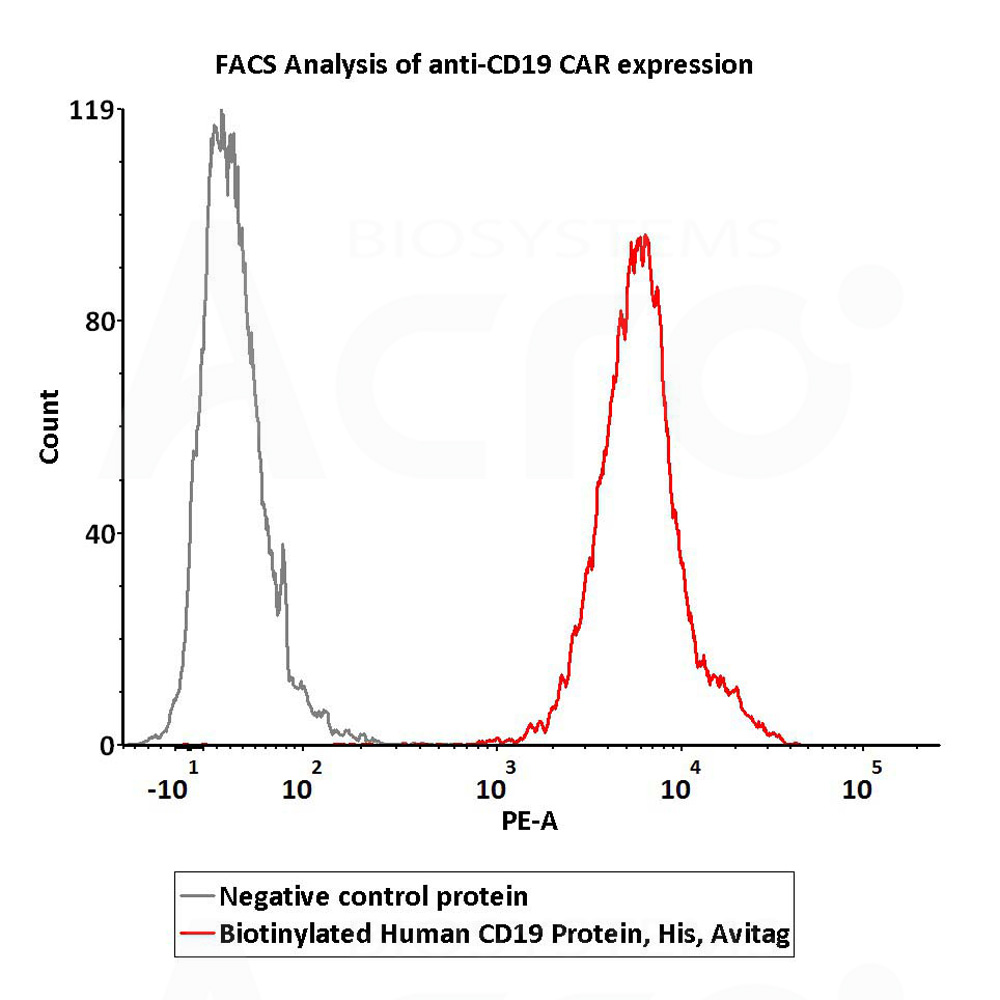
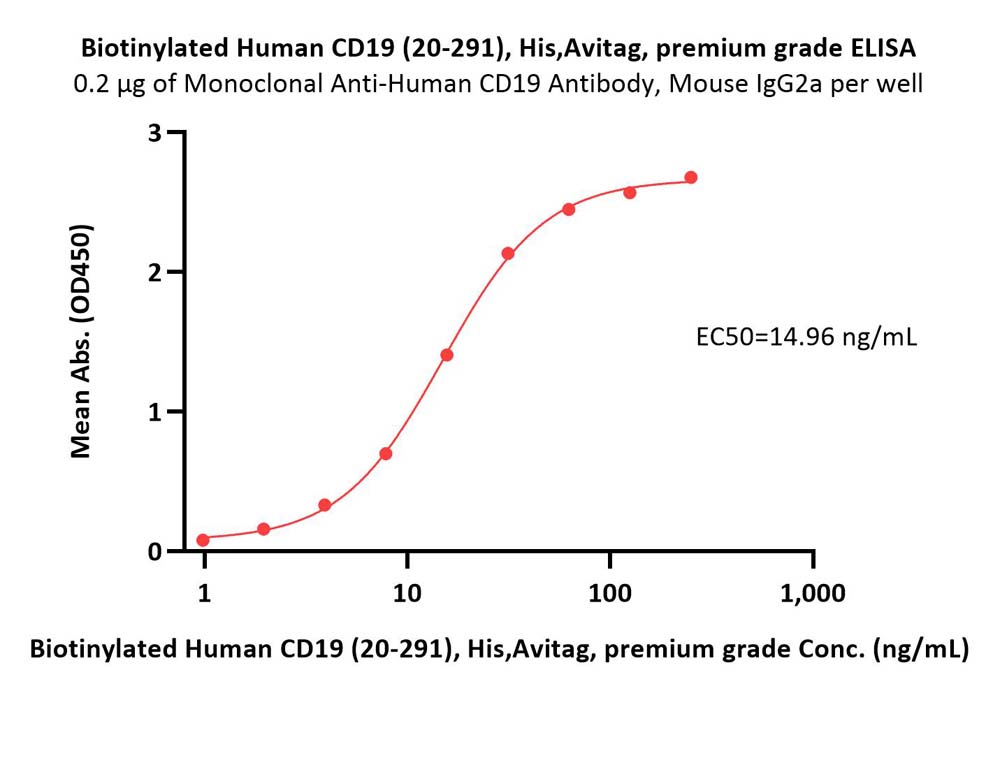
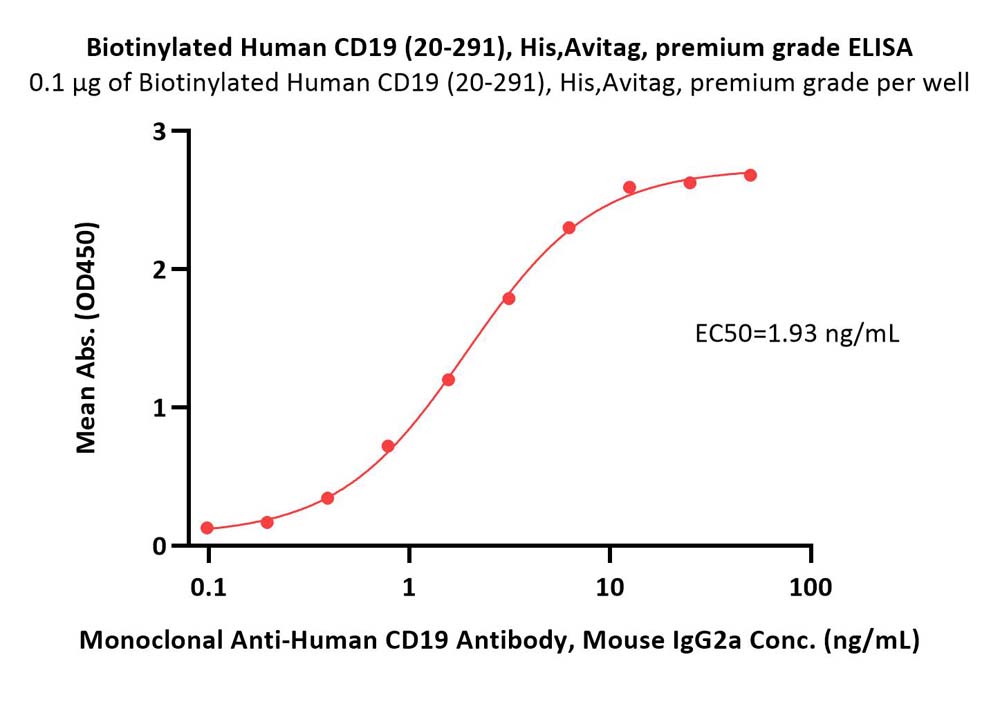
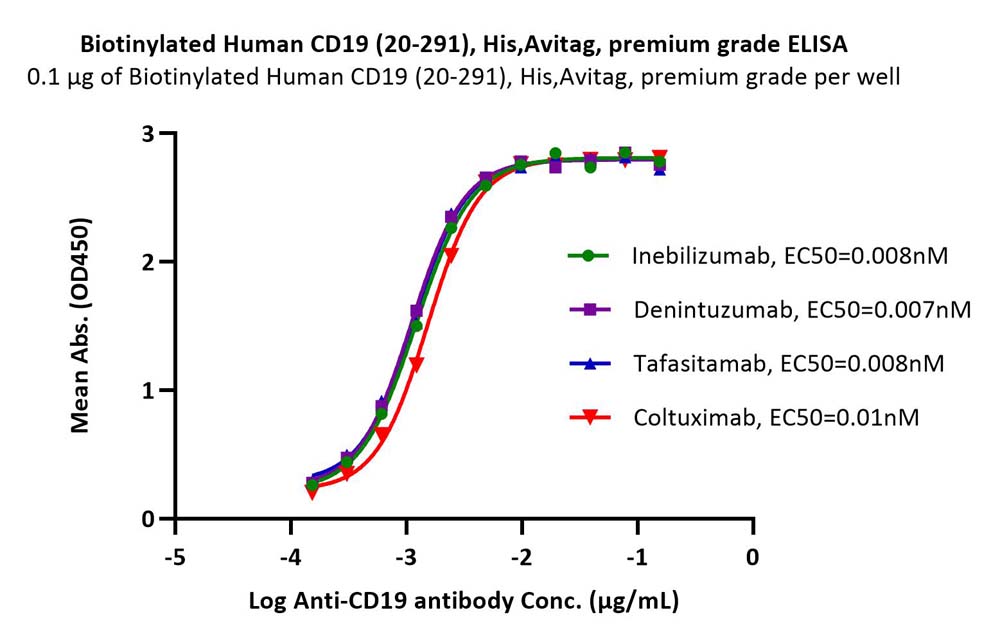
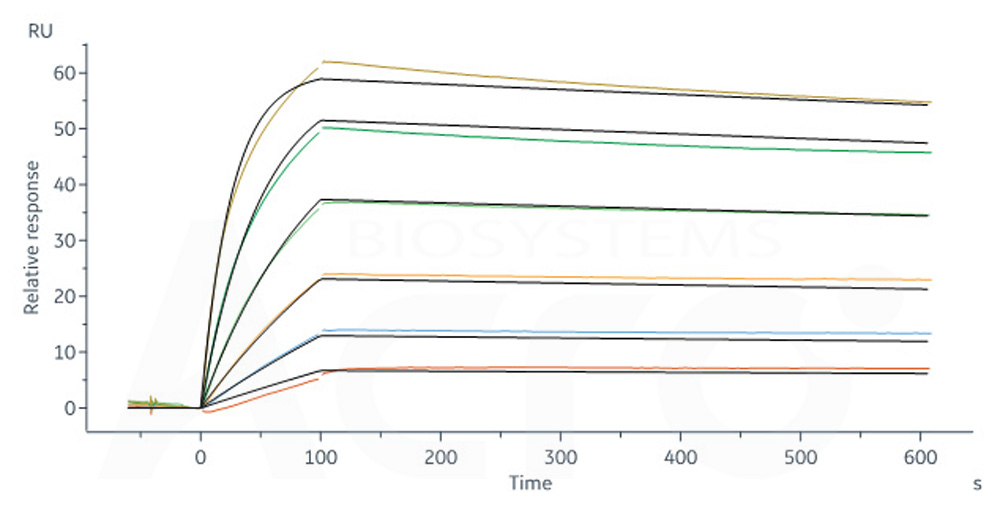
 +添加评论
+添加评论























































 膜杰作
膜杰作 Star Staining
Star Staining